VisCardia has a total of 54 patents globally, out of which 40 have been granted. Of these 54 patents, more than 78% patents are active. United States of America is where VisCardia has filed the maximum number of patents, followed by Europe (EPO) and Germany. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of VisCardia.
VisCardia founded in 2015, VisCardia is a privately held medical device company based in Portland, Oregon, focused on commercializing its proprietary implantable therapy for heart failure. The company is dedicated to advancing innovative solutions in the healthcare and medical device industries. VisCardia’s technology aims to address unmet needs in cardiac care with a novel therapeutic approach.
Do read about some of the most popular patents of VisCardia which have been covered by us in this article and also you can find VisCardia patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over VisCardia patent portfolio.
How many patents does VisCardia have?
VisCardia has a total of 54 patents globally. These patents belong to 5 unique patent families. Out of 54 patents, 42 patents are active.
How Many Patents did VisCardia File Every Year?

Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | VisCardia Applications Filed | VisCardia Patents Granted |
| 2025 | – | 2 |
| 2024 | 1 | 6 |
| 2023 | 2 | 9 |
| 2022 | 1 | 6 |
| 2021 | 7 | 3 |
| 2020 | 7 | 1 |
| 2019 | 3 | 4 |
| 2018 | 1 | 5 |
| 2017 | 23 | 2 |
| 2016 | 2 | 1 |
How many VisCardia patents are Alive/Dead?
Worldwide Patents

How Many Patents did VisCardia File in Different Countries?
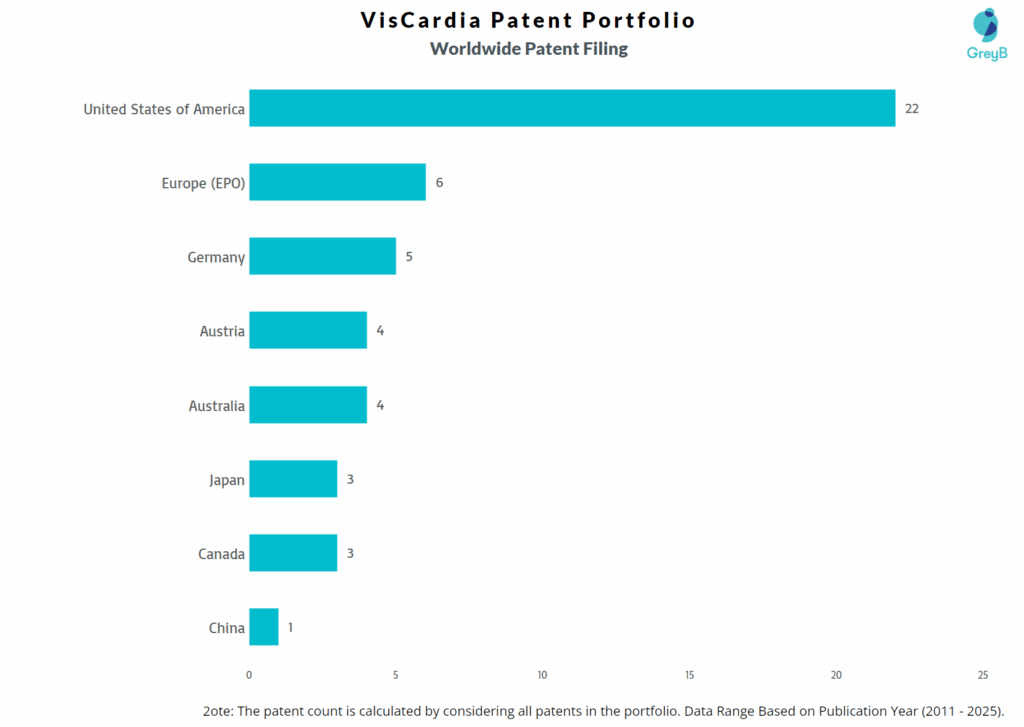
Countries in which VisCardia Filed Patents
| Country | Patents |
| United States of America | 22 |
| Europe (EPO) | 6 |
| Germany | 5 |
| Austria | 4 |
| Australia | 4 |
| Japan | 3 |
| Canada | 3 |
| China | 1 |
Where are Research Centres of VisCardia Patents Located?
All Research centers of VisCardia are in the United States of America.
10 Best VisCardia Patents
US7994655B2 is the most popular patent in the VisCardia portfolio. It has received 53 citations so far from company like Lungpacer Medical.
Below is the list of 10 most cited patents of VisCardia:
| Publication Number | Citation Count |
| US7994655B2 | 53 |
| US9498625B2 | 34 |
| US9968786B2 | 33 |
| US9694185B2 | 33 |
| US10335592B2 | 17 |
| US10369361B2 | 14 |
| US10493271B2 | 9 |
| US10315035B2 | 9 |
| US11925803B2 | 1 |
| US11524158B2 | 1 |
What Percentage of VisCardia US Patent Applications were Granted?
VisCardia (Excluding its subsidiaries) has filed 22 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 20 have been granted leading to a grant rate of 100%.
Below are the key stats of VisCardia patent prosecution at the USPTO.

Which Law Firms are managing US Patents for VisCardia?
| Law Firm | Total Applications | Success Rate |
| Loza & Loza Llp | 22 | 100.00% |
List of VisCardia Patents
| VisCardia Patents | Title |
| EP4034223B1 | Implantable Medical Systems And Devices For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| DE602020044870T2 | Implantable Medical Systems And Devices To Influence The Heart Function Through Diaphragmic Fur Stimulation And To Monitor Diaphragm |
| US12144993B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| US11957914B2 | Implantable Medical Systems, Devices And Methods For Delivering Asymptomatic Diaphragmatic Stimulation |
| US11925803B2 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| US11911616B2 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| JP7527357B2 | Implantable Medical Systems, Devices, And Methods For Influencing Cardiac Function And Monitoring Diaphragm Health Through Diaphragm Stimulation |
| JP7522217B2 | Implantable Medical Systems, Devices And Methods For Delivering Asymptomatic Diaphragm Stimulation |
| US20240207610A1 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| US11684783B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| US11666757B2 | Systems, Devices, And Methods For Improving Hemodynamic Performance Through Asymptomatic Diaphragm Stimulation |
| EP3448501B1 | Implantable Medical Devices For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| EP3448502B1 | Implantable Medical Devices For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| DE602017069512T2 | Implantable Medical Devices For Selecting Optimal Diaphragmatic Stimulation Parameters To Influence Pressures Inside The Intractoracal Cavity |
| DE602017067650T2 | Implantable Medical Devices For Adjusting Diaphramic Stimulation Parameters In Real Time Or Near Real Time To Influence Pressure In The Intrathoracic Cavity |
| AT1573131T | Implantable Medical Devices For Selecting Optimal Diaphragmatic Stimulation Parameters To Influence Pressures Within The Intrathoracic Cavity |
| AT1559454T | Implantable Medical Devices For Adjusting Diaphragmatic Stimulation Parameters In Real Time Or Near Real Time To Influence The Pressure In The Intrathoracic Cavity |
| AU2021269285B2 | Implantable Medical Devices And Methods For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| US20230277842A1 | Systems, Devices, And Methods For Improving Hemodynamic Performance Through Asymptomatic Diaphragm Stimulation |
| EP4126191A1 | Implantable Medical Systems, Devices And Methods For Delivering Asymptomatic Diaphragmatic Stimulation |
| US11524158B2 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| US11458312B2 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| US11400286B2 | Implantable Medical Devices, Systems, And Methods For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| EP3448498B1 | Lead For Implantable Medical Device That Affects Pressures Within The Intrathoracic Cavity Through Diaphragmatic Stimulation |
| DE602017059638T2 | Electrode Lead For Implantable Medical Device Affecting Pressure In The Intrathoracic Cavity By Diaphramic Stimulation |
| AT1505145T | Electrode Lead For Implantable Medical Device With Effect On The Pressure In The Intrathoracic Cavity By Diaphragmatic Stimulation |
| US11147968B2 | Systems, Devices, And Methods For Improving Hemodynamic Performance Through Asymptomatic Diaphragm Stimulation |
| US11020596B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| AU2017258306B2 | Implantable Medical Devices And Methods For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| WO2021194874A1 | Implantable Medical Systems, Devices And Methods For Delivering Asymptomatic Diaphragmatic Stimulation |
| WO2021061793A1 | Implantable Medical Systems, Devices, And Methods For Affecting Cardiac Function Through Diaphragm Stimulation, And For Monitoring Diaphragmatic Health |
| US10537735B2 | Implantable Medical Devices And Methods For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| US10493271B2 | Implantable Medical Devices, Systems, And Methods For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| US10369361B2 | Leads For Implantable Medical Device That Affects Pressures Within The Intrathoracic Cavity Through Diaphragmatic Stimulation |
| US10335592B2 | Systems, Devices, And Methods For Improving Hemodynamic Performance Through Asymptomatic Diaphragm Stimulation |
| US10315035B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| US9968786B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| EP2934668B1 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| DE602013042560T2 | Enhancement Of A Hemodynamic Performance By Asymptomatic Membrane Stimulation |
| AT1031499T | Enhancement Of Hemodynamic Performance Through Asymptomatic Membrane Stimulation |
| JP6285956B2 | Improving Hemodynamic Performance Via Asymptomatic Diaphragmatic Stimulation |
| WO2018067274A1 | Systems, Device, And Methods For Improving Hemodynamic Performance Through Asymptomatic Diaphragm Stimulation |
| AU2017258318A1 | Lead For Implantable Medical Device That Affects Pressures Within The Intrathoracic Cavity Through Diaphragmatic Stimulation |
| AU2017258291A1 | Implantable Medical Devices, Systems, And Methods For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| US9694185B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| CN104994906B | Improvement Of Hemodynamic Function By Asymptomatic Diaphragmatic Stimulation |
| WO2017189895A1 | Implantable Medical Devices And Methods For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| WO2017189907A1 | Lead For Implantable Medical Device That Affects Pressures Within The Intrathoracic Cavity Through Diaphragmatic Stimulation |
| WO2017189880A1 | Implantable Medical Devices, Systems, And Methods For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| CA3022556A1 | Lead For Implantable Medical Device That Affects Pressures Within The Intrathoracic Cavity Through Diaphragmatic Stimulation |
| CA3022495A1 | Implantable Medical Devices, Systems, And Methods For Selection Of Optimal Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| CA3022507A1 | Implantable Medical Devices And Methods For Real-Time Or Near Real-Time Adjustment Of Diaphragmatic Stimulation Parameters To Affect Pressures Within The Intrathoracic Cavity |
| US9498625B2 | Hemodynamic Performance Enhancement Through Asymptomatic Diaphragm Stimulation |
| US7994655B2 | Mechanical, Anatomical Heart-Pumping Assist |
What are VisCardia key innovation segments?
What Technologies are Covered by VisCardia?

The chart below distributes patents filed by VisCardia in different countries on the basis of the technology protected in patents. It also represents the markets where VisCardia thinks it’s important to protect particular technological inventions.

R&D Focus: How has VisCardia search focus changed over the years?

EXCLUSIVE INSIGHTS COMING SOON!
Interested in knowing about the areas of innovation that are being protected by VisCardia?

